What is our assessment?
Based on the scientific uncertainties regarding its effectiveness and the potential serious environmental and social risks, we conclude that Deploy Ocean Fertilization is “Not Recommended” as a climate solution.
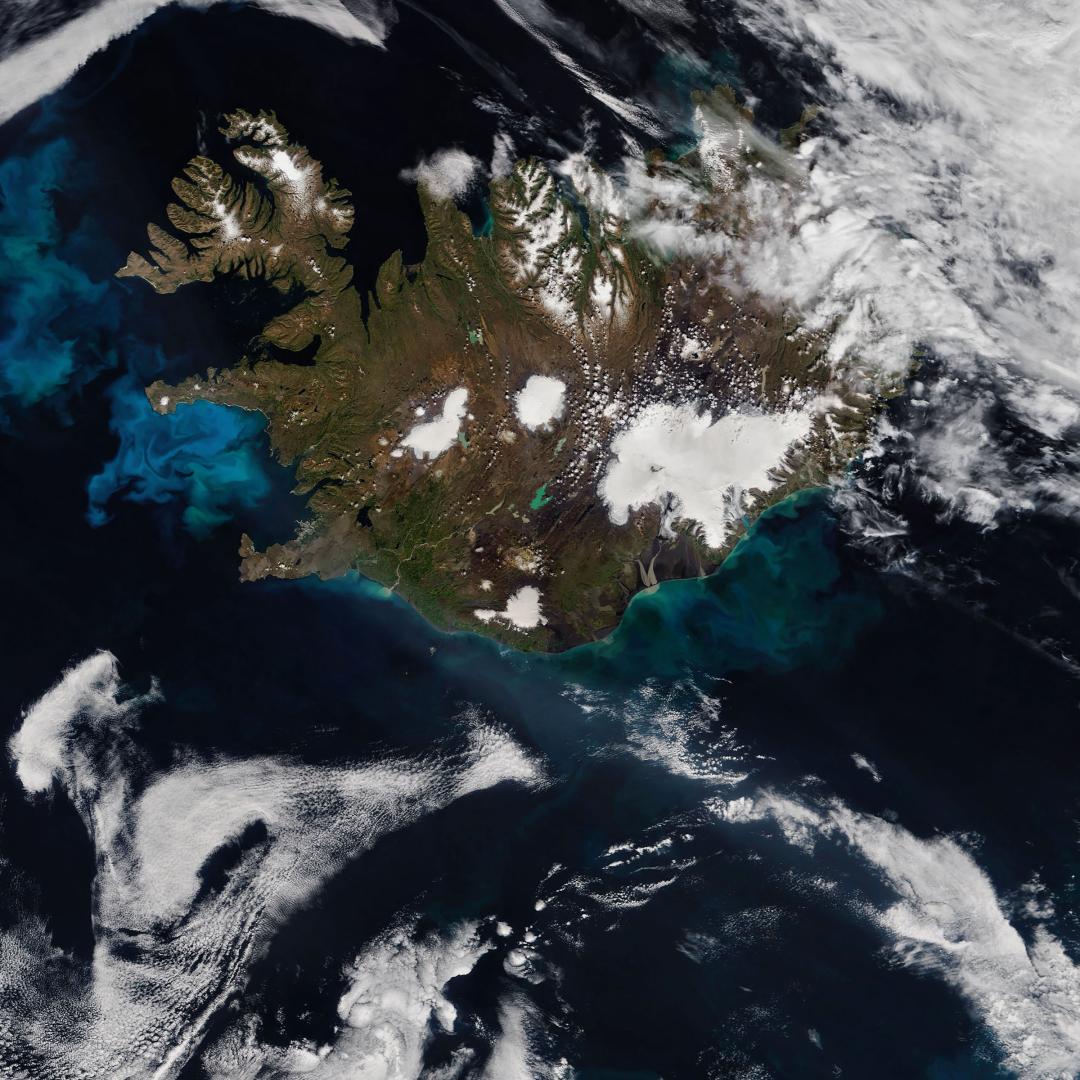
Ocean fertilization uses nutrients to enhance photosynthesis by marine phytoplankton, which remove CO₂ and convert it into biomass that can sink to the deep ocean. This practice is a carbon removal technology that could achieve Gt-scale CO₂ removal annually. Potential advantages of ocean fertilization include localized reduction of ocean acidification and low costs. Disadvantages include high and uncertain risks of altering ecosystems both near dispersal sites and farther away, unclear but probably low effectiveness, potentially difficult operational upscaling, and challenges with monitoring and verification. We conclude that Deploy Ocean Fertilization is “Not Recommended” as a climate solution given its likely low effectiveness, technical challenges, and high environmental risks.
Based on the scientific uncertainties regarding its effectiveness and the potential serious environmental and social risks, we conclude that Deploy Ocean Fertilization is “Not Recommended” as a climate solution.
| Plausible | Could it work? | Yes |
|---|---|---|
| Ready | Is it ready? | No |
| Evidence | Are there data to evaluate it? | Limited |
| Effective | Does it consistently work? | No |
| Impact | Is it big enough to matter? | Yes |
| Risk | Is it risky or harmful? | Yes |
| Cost | Is it cheap? | ? |
Ocean fertilization involves adding nutrients, such as iron, to seawater to promote photosynthesis in the surface ocean. As phytoplankton draw in seawater CO₂ and convert it into biomass, the ocean can absorb more CO₂ from the atmosphere. Some of the carbon eventually sinks or is transported to the deep sea or seafloor, where it can be stored for decades or centuries. Most ocean fertilization efforts are focused on adding iron because it is a micronutrient already required in small amounts for photosynthesis and because iron limitation is common in many global ocean regions. The Southern Ocean, in particular, has been highlighted as a potential target due to its widespread iron limitation.
As a carbon removal technique, ocean fertilization requires that the nutrient addition enhances phytoplankton uptake of seawater CO₂ and subsequent absorption of additional CO₂ from the atmosphere, and that the carbon is transported and durably stored in the deep sea. Research since the 1990s has shown that ocean iron fertilization does lead to increased seawater CO₂ uptake due to enhanced photosynthesis. However, the ultimate fate and durability of that carbon are less well understood. To be sequestered, carbon must be transported below water depths where annual mixing occurs, often considered to be ~1,000 m, but research suggests that, on average, 66% of carbon at these depths can be re-exposed to the atmosphere in less than 40 years. Ocean fertilization might also increase production of GHGs, such as nitrous oxide and methane, which could impact the effectiveness of this practice, although these effects remain understudied. In places like the Southern Ocean, sunlight and changes in the availability of other nutrients, such as silicate, can also limit the effects of iron addition. Additionally, nutrients such as iron can have high loss rates, up to 75%, after dispersal into seawater due to conversion into forms inaccessible to phytoplankton, potentially further reducing the effectiveness of nutrient addition.
If ocean fertilization were broadly deployed and functioned as intended, its global climate impact could reach 0.1–1.0 Gt CO₂ /yr. Ocean fertilization is expected to increase surface water pH, which could help temporarily reduce ocean acidification locally. However, some studies suggest this benefit will come at the cost of increased acidification of deeper ocean regions. While costs remain highly uncertain, estimates of ocean fertilization costs range between US$80/t CO₂ and US$457/t CO₂, suggesting this practice might also be relatively inexpensive compared to other marine CO₂ removal practices.
Ocean fertilization poses several technical challenges, along with significant environmental and social risks. Tracking the amount of carbon sequestered from ocean fertilization is difficult because carbon export efficiencies – the amount of carbon produced in surface waters that makes its way to the deep sea – can be low and highly variable in time and space. Addressing this will require both field studies and models capable of capturing global and multi-decadal changes in carbon cycling due to fertilization, given the long time scales and large spatial areas involved. Implementing ocean fertilization at globally meaningful carbon removal levels could raise additional feasibility concerns, given the potential difficulty of dispersing sufficiently large quantities of nutrients across vast areas and the need for fertilization to be done continuously to minimize carbon returning to the atmosphere.
Beyond these technical challenges, ocean fertilization also poses several potentially severe environmental risks. Enhancing primary production could disrupt existing nutrient pools in the ocean, reducing the nutrients available for ecosystems far from dispersal sites. Another consequence of ocean fertilization is that increased organic carbon supply can enhance microbial processes that consume dissolved oxygen, potentially impairing respiration in marine organisms and leading to mortality. Other unintended consequences of nutrient fertilization include promoting harmful algal blooms that can release toxins that negatively impact a wide array of life, from shellfish to marine mammals to humans. Ocean fertilization also carries significant social risks because global-scale modification of marine ecosystems is likely to create inequities in environmental and economic impacts.
Aumont, O., & Bopp, L. (2006). Globalizing results from ocean in situ iron fertilization studies. Global Biogeochemical Cycles, 20(2). Link to source: https://doi.org/10.1029/2005GB002591
Bakker, D. C. (2004). Storage of carbon dioxide by greening of oceans. In C. B. Field & M. R. Raupach (Eds.), The global carbon cycle: Integrating humans, climate, and the natural world (pp. 453–469). Island Press.
Boettcher, M., Chai, F., Canothan, M., Cooley, S., Keller, D. P., Klinsky, S., ... & Webb, R. M. (2023). A code of conduct for marine carbon dioxide removal research. Aspen Institute. Link to source: https://www.aspeninstitute.org/publications/a-code-of-conduct-for-marine-carbon-dioxide-removal-research/
Boyd, P. W. (2008). Implications of large-scale iron fertilization of the oceans. Marine Ecology Progress Series, 364, 213–218. Link to source: https://www.int-res.com/articles/theme/m364p213.pdf
Boyd, P. W., Jickells, T., Law, C. S., Blain, S., Boyle, E. A., Buesseler, K. O., ... & Watson, A. J. (2007). Mesoscale iron enrichment experiments 1993-2005: synthesis and future directions. Science, 315(5812), 612–617. Link to source: https://doi.org/10.1126/science.1131669
Buesseler, K. O., & Boyd, P. W. (2003). Will ocean fertilization work?. Science, 300(5616), 67–68. Link to source: https://doi.org/10.1126/science.1082959
Cao, L., & Caldeira, K. (2010). Can ocean iron fertilization mitigate ocean acidification? A letter. Climatic Change, 99(1), 303–311. Link to source: https://link.springer.com/article/10.1007/s10584-010-9799-4
Emerson, D., Sofen, L. E., Michaud, A. B., Archer, S. D., & Twining, B. S. (2024). A cost model for ocean iron fertilization as a means of carbon dioxide removal that compares ship‐and aerial‐based delivery, and estimates verification costs. Earth's Future, 12(4), e2023EF003732. Link to source: https://doi.org/10.1029/2023EF003732
Gattuso, J. P., Williamson, P., Duarte, C. M., & Magnan, A. K. (2021). The potential for ocean-based climate action: negative emissions technologies and beyond. Frontiers in Climate, 2, 575716. Link to source: https://doi.org/10.3389/fclim.2020.575716
Harrison, D. P. (2013). A method for estimating the cost to sequester carbon dioxide by delivering iron to the ocean. International Journal of Global Warming, 5(3), 231–254. Link to source: https://doi.org/10.1504/IJGW.2013.055360
Harvey, J. (2020). 30 years: The iron hypothesis is no more. Moss Landing Marine Laboratories Blog. Link to source: https://mlml.sjsu.edu/2020/06/18/30-years-the-iron-hypothesis-is-no-more/
Jin, X., & Gruber, N. (2003). Offsetting the radiative benefit of ocean iron fertilization by enhancing N2O emissions. Geophysical Research Letters, 30(24). Link to source: https://doi.org/10.1029/2003GL018458
Marinov, I., Gnanadesikan, A., Toggweiler, J. R., & Sarmiento, J. L. (2006). The southern ocean biogeochemical divide. Nature, 441(7096), 964–967. Link to source: https://doi.org/10.1038/nature04883
Martin, J. H., Gordon, M., & Fitzwater, S. E. (1991). The case for iron. Limnology and Oceanography, 36(8), 1793–1802. Link to source: https://doi.org/10.4319/lo.1991.36.8.1793
National Academies of Sciences, Engineering, and Medicine. (2021). A research strategy for ocean-based carbon dioxide removal and sequestration. National Academies Press. Link to source: https://www.nationalacademies.org/our-work/a-research-strategy-for-ocean-carbon-dioxide-removal-and-sequestration
Ocean Visions. (2023). Microalgae cultivation. Link to source: https://oceanvisions.org/microalgae-cultivation/
Oschlies, A., Koeve, W., Rickels, W., & Rehdanz, K. (2010). Side effects and accounting aspects of hypothetical large-scale Southern Ocean iron fertilization. Biogeosciences, 7(12), 4017–4035. Link to source: https://bg.copernicus.org/articles/7/4017/2010/bg-7-4017-2010.pdf
Oschlies, A., Slomp, C., Altieri, A. H., Gallo, N. D., Grégoire, M., Isensee, K., Levin, L. A., & Wu, J. (2025). Potential impacts of marine carbon dioxide removal on ocean oxygen. Environmental Research Letters, 20(1), 011001. Link to source: https://doi.org/10.1088/1748-9326/ade0d4
Robinson, J., Popova, E. E., Yool, A., Srokosz, M., Lampitt, R. S., & Blundell, J. R. (2014). How deep is deep enough? Ocean iron fertilization and carbon sequestration in the Southern Ocean. Geophysical Research Letters, 41(7), 2489–2495. Link to source: https://doi.org/10.1002/2013GL058799
Sarmiento, J. L., Gruber, N., Brzezinski, M. A., & Dunne, J. P. (2004). High-latitude controls of thermocline nutrients and low latitude biological productivity. Nature, 427(6969), 56–60. Link to source: https://doi.org/10.1038/nature02127
Shepherd, J. G. (2009). Geoengineering the climate: science, governance and uncertainty. The Royal Society. Link to source: https://royalsociety.org/-/media/policy/publications/2009/8693.pdf
Strong, A., Chisholm, S., Miller, C., & Cullen, J. (2009). Ocean fertilization: time to move on. Nature, 461(7262), 347–348. Link to source: https://doi.org/10.1038/461347a
Tagliabue, A., Aumont, O., DeAth, R., Dunne, J. P., Dutkiewicz, S., Galbraith, E., Misumi, K., Moore, J. K., Ridgwell, A., Sherman, E., Stock, C., Vichi, M., Völker, C., & Yool, A. (2016). How well do global ocean biogeochemistry models simulate dissolved iron distributions?. Global Biogeochemical Cycles, 30(2), 149–174. Link to source: https://doi.org/10.1002/2015GB005289
Tagliabue, A., Twining, B. S., Barrier, N., Maury, O., Berger, M., & Bopp, L. (2023). Ocean iron fertilization may amplify climate change pressures on marine animal biomass for limited climate benefit. Global Change Biology, 29(18), 5250–5260. Link to source: https://doi.org/10.1111/gcb.16854
Trick, C. G., Bill, B. D., Cochlan, W. P., Wells, M. L., Trainer, V. L., & Pickell, L. D. (2010). Iron enrichment stimulates toxic diatom production in high-nitrate, low-chlorophyll areas. Proceedings of the National Academy of Sciences, 107(13), 5887–5892. Link to source: https://doi.org/10.1073/pnas.0910579107
Yoon, J. E., Yoo, K. C., Macdonald, A. M., Yoon, H. I., Park, K. T., Yang, E. J., Kim, H. C., Lee, J. I., Lee, M. K., Jung, J., Park, J., Lee, J., Kim, S., Kim, S. S., Kim, K., & Kim, I. N. (2018). Reviews and syntheses: Ocean iron fertilization experiments–past, present, and future looking to a future Korean Iron Fertilization Experiment in the Southern Ocean (KIFES) project. Biogeosciences, 15(19), 5847-5889. Link to source: https://doi.org/10.5194/bg-15-5847-2018



Vertical farms are facilities that grow crops indoors, vertically stacking multiple layers of plants and providing controlled conditions using artificial light, indoor heating and cooling systems, humidity controls, water pumps, and advanced automation systems. In theory, vertical farms could reduce the need to clear more agricultural land and the distance food travels to market. However, because vertical farms are so energy and material intensive, and food transportation emissions are a small fraction of the overall carbon footprint of food, vertical farms do not reduce emissions overall. We conclude that vertical farms are “Not Recommended” as an effective climate solution.
Based on our analysis, vertical farms are not an effective climate solution. The tremendous energy use and embodied emissions of vertical farm operations outweigh any potential savings of reducing food miles or land expansion. Moreover, the ability of vertical farms to truly scale to be a meaningful part of the global food system is extremely limited. We therefore classify this as “Not Recommended” as an effective climate solution.
| Plausible | Could it work? | No |
|---|---|---|
| Ready | Is it ready? | Yes |
| Evidence | Are there data to evaluate it? | Yes |
| Effective | Does it consistently work? | No |
| Impact | Is it big enough to matter? | No |
| Risk | Is it risky or harmful? | No |
| Cost | Is it cheap? | No |
Vertical farms are facilities that grow crops indoors, with multiple layers of plants stacked on top of each other, using artificial lights, large heating and cooling systems, humidity controls, water pumps, and complex building automation systems. In principle, vertical farms can dramatically shrink the land “footprint” of agriculture, and this could help reduce the need for agricultural land. Moreover, by growing crops closer to urban centers, vertical farms could potentially reduce “food miles” and the emissions related to food transport.
The technology of growing some kinds of crops – especially greens and herbs – in indoor facilities is well developed, but there is no evidence to show that doing so can reduce GHG emissions compared to growing the same food on traditional farms. Theoretically, vertical farms could reduce emissions associated with agricultural land expansion and food transportation. However, the operation and construction of vertical farms require enormous amounts of energy and materials, all of which cause significant emissions. Vertical farms require artificial lighting (even with efficient LEDs, this is a considerable energy cost), heating, cooling, humidity control, air circulation, and water pumping – all of which require energy. Vertical farms could be powered by renewable sources; however, this is an inefficient method for reducing GHG emissions compared to using that renewable energy to replace fossil-fuel-powered electricity generation. Growing food closer to urban centers also does not meaningfully reduce emissions because emissions from “food miles” are only a small fraction of the life cycle emissions for most farmed foods. Recent research has found that the carbon footprint of lettuce grown in vertical farms can be 5.6 to 16.7 times greater than that of lettuce grown with traditional methods.
While vertical farms are not an effective strategy for reducing emissions, they may have some value for climate resilience and adaptation. Vertical farms offer a protected environment for crop growth and well-managed water use, and they can potentially shield plants from pests, diseases, and natural disasters. Moreover, the controlled environment can be adjusted to adapt to changing climate conditions, helping ensure continuous production and lowering the risks of crop loss.
Vertical farms use enormous amounts of energy and material to grow a limited array of food, all at significant cost. That energy and material have a significant carbon emissions cost, no matter how efficient the technology becomes. On the whole, vertical farms appear to emit far more GHGs than traditional farms do. Moreover, vertical farms are expensive to build and operate, and are unlikely to play a major role in the world’s food system. At present, they are mainly used to grow high-priced greens, vegetables, herbs, and cannabis, which do not address the tremendous pressure points in the global food system to feed the world sustainably. There are also concerns about the future of the vertical farming business. While early efforts were funded by venture capital, vertical farming has struggled to become profitable, putting its future in doubt.
Blom, T. et al.., (2022). The embodied carbon emissions of lettuce production in vertical farming, greenhouse horticulture, and open-field farming in the Netherlands. Journal of Cleaner Production, 377, 134443. Link to source: https://www.sciencedirect.com/science/article/pii/S095965262204015X
Cornell Chronicle, (2014). Indoor urban farms called wasteful, “pie in the sky.” Link to source: https://news.cornell.edu/stories/2014/02/indoor-urban-farms-called-wasteful-pie-sky
Cox, S., (2012). The vertical farming scam, Counterpunch. Link to source: https://www.counterpunch.org/2012/12/11/the-vertical-farming-scam/
Cox, S., (2016). Enough with the vertical farming fantasies: There are still too many unanswered questions about the trendy practice, Salon. Link to source: https://www.salon.com/2016/02/17/enough_with_the_vertical_farming_partner/
Foley, J.A. et al., (2011). Solutions for a cultivated planet, Nature. Link to source: http://doi.org/10.1038/nature10452
Foley, J.A., (2018). No, vertical farms won’t feed the world, Medium. Link to source: https://globalecoguy.org/no-vertical-farms-wont-feed-the-world-5313e3e961c0
Hamm, M., (2015). The buzz around indoor farms and artificial lighting makes no sense. The Guardian. Link to source: https://www.theguardian.com/sustainable-business/2015/apr/10/indoor-farming-makes-no-economic-environmental-sense
Peters, A., (2023). The vertical farming bubble is finally popping, Fast Company. Link to source: https://www.fastcompany.com/90824702/vertical-farming-failing-profitable-appharvest-aerofarms-bowery
Ritchie, H., (2022). Eating local is still not a good way to reduce the carbon footprint of your diet, Sustainability by the numbers. Link to source: https://www.sustainabilitybynumbers.com/p/food-miles
Tabibi, A. (2024). Vertical farms: A tool for climate change adaptation, Green.org. January 30, 2024. Link to source: https://green.org/2024/01/30/vertical-farms-a-tool-for-climate-change-adaptation/

Corn ethanol, an alcohol made by fermenting corn grain, is the most produced and used biofuel in the United States. The U.S. Renewable Fuel Standard requires that corn ethanol be blended with gasoline for the intended purpose of reducing transportation emissions. Ethanol is a useful vehicle fuel additive that improves engine performance and reduces air pollution. However, life cycle emissions analyses show that corn ethanol does not reduce GHG emissions as claimed and, more likely, increases emissions by 24% compared to gasoline alone. One-third of the corn grown in the U.S. is now used to produce more than 15 billion gallons of ethanol per year. This huge demand for corn has increased prices and driven the conversion of unfarmed land and natural ecosystems. The higher demand for corn also led to more fertilizer use on farms, resulting in increased pollution and nitrous oxide emissions. Based on these life cycle analyses, we conclude that using corn ethanol is "Not Recommended" as a climate solution.
The use of corn ethanol as a transportation biofuel, which has led to the expansion and intensification of corn production, does not reduce GHG emissions compared to gasoline. Based on this finding, using corn ethanol is not a plausible approach for reducing emissions and is “Not Recommended” as a climate solution.
| Plausible | Could it work? | No |
|---|---|---|
| Ready | Is it ready? | Yes |
| Evidence | Are there data to evaluate it? | Yes |
| Effective | Does it consistently work? | No |
| Impact | Is it big enough to matter? | No |
| Risk | Is it risky or harmful? | No |
| Cost | Is it cheap? | No |
Corn ethanol is a liquid biofuel that is blended with gasoline to displace a fraction of the petroleum-based fuel with a renewable fuel derived from plants. Proponents claim that blending corn ethanol with gasoline reduces emissions because the CO₂ produced from combusting the ethanol is offset, or balanced out, by the atmospheric CO₂ absorbed by the corn plant during growth. Corn ethanol is made from corn grain by breaking down the starch in the kernels into sugar and then fermenting it into a liquid. In the United States, the world leader in biofuel production, almost 90% of biofuel is corn ethanol. Most gasoline now sold in the U.S. contains about 10% corn ethanol, and, in 2025, the Renewable Fuel Standard (RFS) program requires production of more than 15 billion gallons of this biofuel. Currently, it is primarily made from corn kernels; the technology for producing biomass-derived ethanol from other, non-edible parts of the corn plant is not yet commercially viable. Brazil is the second-largest producer of ethanol, but uses sugarcane as a feedstock.
The Renewable Fuel Standard requires that the life cycle emissions from corn ethanol be at least 20% lower than those of conventional gasoline. However, based on comprehensive life cycle emissions analyses, using corn ethanol does not reduce emissions compared to gasoline. The main reasons for this are that the production of corn and processing it into ethanol generate large amounts of emissions, including from land conversion, fertilizer-related nitrous oxide emissions, and the industrial process of fermenting the corn into ethanol. The most prominent recent study reported that corn ethanol life cycle emissions were, at best, no less than gasoline and, more likely, were 24% higher. Corn ethanol is also more emissions-intensive than ethanol made from other plants, like sugar cane.
Ethanol has been used as a transportation fuel, including as a blend with gasoline, for more than a century. It boosts the octane number of fuel, improves engine performance and fuel economy, and reduces emissions of harmful pollutants like unburned hydrocarbons, nitrogen oxides, and particulates. Ethanol has also been used to replace other harmful and polluting gasoline additives, including lead and methyl tert-butyl ether (MTBE). Ethanol produced from non-edible biological feedstocks with lower production emissions, such as switchgrass or cellulose from crop residues, has the potential to reduce emissions.
The Renewable Fuel Standard (RFS) program requires that biofuels be blended into the transportation fuel supply at annually increasing increments. The United States now uses one-third of its corn to generate more than 15 billion gallons of ethanol per year. Not only does this mandated program not reduce emissions (it more likely increases emissions), but it also consumes corn that could otherwise be used for food or animal feed. The increased demand for corn for ethanol has increased corn prices, which in turn have contributed to the conversion of grasslands and semi-natural ecosystems to grow more corn. When grasslands, woodlands, or other natural ecosystems are plowed and converted to cropland, the carbon stored in the vegetation and soil is emitted to the atmosphere. Between 2008 and 2016, the conversion of 1.8 Mha of natural and semi-natural land in the U.S. released about 400 million metric tons of CO₂ from vegetation and soil. The increased corn production also increased the application of synthetic fertilizers, which has increased nitrate leaching, phosphorus runoff, and emissions of nitrous oxide, a powerful GHG (see Improve Nutrient Management). These problems are particularly severe in the U.S. Midwest and the Mississippi River drainage.
Broda, M., Yelle, D. J., & Serwańska, K. (2022). Bioethanol production from lignocellulosic biomass—challenges and solutions. Molecules, 27(24), 8717. Link to source: https://www.mdpi.com/1420-3049/27/24/8717
California Air Resources Board (CARB) (2003). Cleaner Burning Gasoline without MTBE. Link to source: https://ww2.arb.ca.gov/resources/fact-sheets/cleaner-burning-gasoline-without-mtbe
Cassidy, E. (2014). Ethanol’s Broken Promise. Environmental Working Group. Link to source: https://www.ewg.org/research/ethanols-broken-promise
Ciolkosz, D. (2024). Fuel Ethanol: Hero or Villain? Penn State Extension. Link to source: https://extension.psu.edu/fuel-ethanol-hero-or-villain
Douglas, L. (2022). U.S. corn-based ethanol worse for the climate than gasoline, study finds. Reuters. Link to source: https://www.reuters.com/business/environment/us-corn-based-ethanol-worse-climate-than-gasoline-study-finds-2022-02-14/
EPA (U.S. Environmental Protection Agency) (2023). Renewable Fuel Standard (RFS) Program: Standards for 2023–2025 and Other Changes Lifecycle Greenhouse Gas Results. Federal Register/Vol. 88, No. 132/Wednesday, July 12, 2023/Rules and Regulations. Link to source: https://www.govinfo.gov/content/pkg/FR-2023-07-12/pdf/2023-13462.pdf
EPA (U.S. Environmental Protection Agency) (2025a). Overview of the Renewable Fuel Standard Program. Link to source: https://www.epa.gov/renewable-fuel-standard/overview-renewable-fuel-standard-program
EPA (U.S. Environmental Protection Agency) (2025b). Lifecycle Greenhouse Gas Results. Link to source: https://www.epa.gov/fuels-registration-reporting-and-compliance-help/lifecycle-greenhouse-gas-results
Hill, J. (2022). The sobering truth about corn ethanol. Proceedings of the National Academy of Sciences, 119(11), e2200997119. Link to source: https://doi.org/10.1073/pnas.2200997119
Kramer, D. (2022). Whatever happened to cellulosic ethanol? Physics Today, 75(7), 22-24. Link to source: https://doi.org/10.1063/PT.3.5036
Lark, T. J., Hendricks, N. P., Smith, A., Pates, N., Spawn-Lee, S. A., Bougie, M., ... & Gibbs, H. K. (2022). Environmental outcomes of the US renewable fuel standard. Proceedings of the National Academy of Sciences, 119(9), e2101084119. Link to source: https://doi.org/10.1073/pnas.2101084119
Lark, T. J., Salmon, J. M., & Gibbs, H. K. (2015). Cropland expansion outpaces agricultural and biofuel policies in the United States. Environmental Research Letters, 10(4), 044003. Link to source: http://dx.doi.org/10.1088/1748-9326/10/4/044003
National Library of Medicine (2023). Toxicological Profile for Methyl tert-Butyl Ether (MTBE). Agency for Toxic Substances and Disease Registry (US); CHAPTER 1, RELEVANCE TO PUBLIC HEALTH. Link to source: https://www.ncbi.nlm.nih.gov/books/NBK601216/
Robertson, G. P., Dale, V. H., Doering, O. C., Hamburg, S. P., Melillo, J. M., Wander, M. M., ... & Wilhelm, W. W. (2008). Sustainable biofuels redux. Science, 322(5898), 49–50. Link to source: https://lter.kbs.msu.edu/docs/robertson/robertson_et_al._2008_science.pdf
Searchinger, T. et al. (2008) Use of U.S. Croplands for Biofuels Increases Greenhouse Gases Through Emissions from Land-Use Change. Science 319,1238-1240(2008). Link to source: https://doi.org/10.1126/science.1151861
Spawn, S. A., Lark, T. J., & Gibbs, H. K. (2019). Carbon emissions from cropland expansion in the United States. Environmental Research Letters, 14(4), 045009. Link to source: https://doi.org/10.1088/1748-9326/ab0399
Tilman, D., Socolow, R., Foley, J. A., Hill, J., Larson, E., Lynd, L., ... & Williams, R. (2009). Beneficial biofuels—the food, energy, and environment trilemma. Science, 325(5938), 270-271. Link to source: https://doi.org/10.1126/science.1177970
Wright, C. K., Larson, B., Lark, T. J., & Gibbs, H. K. (2017). Recent grassland losses are concentrated around US ethanol refineries. Environmental Research Letters, 12(4), 044001. Link to source: https://doi.org/10.1088/1748-9326/aa6446


Carbon capture and storage (CCS) reduces the operational GHG emissions from fossil fuel power plants by selectively capturing CO₂ from the plant’s exhaust flue, preventing it from entering the atmosphere. The captured CO₂ is then concentrated, compressed, and permanently stored underground. The carbon capture technology is effective and available, but it is expensive and energy-intensive. Globally, emissions from coal and gas power plants are still increasing, potentially making retrofitting newer plants with CCS an appealing emissions reduction strategy. However, despite 30 years of pilot and commercial projects, most power plant CCS projects have failed. While CCS can cut CO₂ emissions, large-scale deployment of this technology on fossil-fueled power plants will likely drive continued production and use of coal and gas. Based on this risk, as well as the availability of cheaper, clean energy alternatives for power generation, we conclude that using CCS on fossil fuel power plants is “Not Recommended” as a climate solution.
Using CCS on fossil-fueled power plants will reduce electricity production emissions, but it is more expensive, more energy-intensive, and more polluting than readily available, cheaper, and cleaner alternatives like wind, solar, and geothermal. Based on this, and the risk that large-scale deployment of CCS on fossil-fueled power plants could drive continued production and use of coal and gas, we conclude that using CCS on fossil fuel power plants is “Not Recommended” as a climate solution.
| Plausible | Could it work? | Yes |
|---|---|---|
| Ready | Is it ready? | Yes |
| Evidence | Are there data to evaluate it? | Yes |
| Effective | Does it consistently work? | No |
| Impact | Is it big enough to matter? | Yes |
| Risk | Is it risky or harmful? | Yes |
| Cost | Is it cheap? | No |
Carbon capture and storage (CCS) is a technology that reduces GHG emissions from fossil fuel-powered electricity generation facilities by selectively capturing CO₂ from the power plant’s exhaust flue, preventing it from entering the atmosphere. The captured CO₂ is then concentrated, compressed, and permanently stored underground. There are other commercially available CCS technologies, such as pre-combustion capture and oxy-fuel combustion, but these are used almost exclusively for industrial processes like gas processing and cannot be readily retrofitted to existing power plants. CCS can also be applied to capture CO₂ from other industrial facilities that generate emissions from fuel combustion or production processes, like cement or ethanol production plants, or from biomass energy power plants. Instead of permanent storage, captured CO₂ can also be used as a chemical precursor for the manufacture of other products or for enhanced oil recovery, but, compared with geologic storage, these post-capture uses of CO₂ emit GHGs, thereby reducing or eliminating the emissions reduction efficacy of CCS.
The technology and chemistry for the selective capture of CO₂ from the exhaust of a power plant are effective. There are numerous chemical, membrane, and cryogenic methods for capturing CO₂, but monoethanolamine (MEA) is the predominant commercially available chemical absorbent currently in use in power plants with CCS. CO₂ capture efficiency varies with the type of reactive absorbent material and plant operations. Most CCS installations target 90% CO₂ capture rates, although actual capture rates are usually lower. CCS infrastructure is large, and the process of capturing CO₂ from power plant exhaust is complex, expensive, and energy-intensive. CCS requires the flue gas to be pumped to different parts of the power plant, the CO₂ to be captured and then separated from the sorbent material, and the concentrated CO₂ to be compressed for transport and storage. Energy for all these processes comes from the power plant. Various studies estimate CCS consumes at least 15–25% of the plant’s total generation capacity, with most of the energy used to separate the CO₂ and regenerate the sorbent material.
CCS has been used in pilot studies and commercial operations in a few dozen coal and natural gas power plants since the late 1990s. Despite the functional effectiveness of the technology, use of CCS to reduce power plant emissions has not been broadly adopted, and most CCS projects initiated in the past three decades have failed or been discontinued. Based on various assessments and projections, deployment of CCS on power plants has consistently lagged behind its expected contribution to emissions reduction. There are currently only four power plants with CCS in operation in the world, less than 0.05% of the global fossil-fueled power plant fleet. According to a 2021 study, only 10% of proposed CCS projects for power plants have actually been implemented. Based on another study, 78% of all power plant and industrial manufacturing CCS pilot and demonstration plants with a project size greater than 0.3 Mt CO₂ /yr have been cancelled or put on hold.
Globally, emissions from coal- and gas-fired power plants are still increasing, primarily in China and India, where large numbers of new thermal power plants have been built in the last two decades. Given the typical 30- to 45-year operational lifespan for coal and gas power plants, retrofitting these newer plants with CCS could substantially reduce their operational emissions while also allowing plant owners and investors to recover their investments. Installation of CCS to reduce emissions can also be prioritized for power plants located near places with geologic storage and where alternative electricity generation options are limited. There is a large amount of research underway to develop and test alternative carbon capture technologies, most aimed at increasing carbon capture efficiencies and reducing energy demands and costs. Other research on the factors contributing to the failure of most CCS projects to date may lead to the development of regulations and policies that require or incentivize the use of CCS for power plants, which could increase the current low implementation and success rates for this emissions reduction technology.
While CCS can reduce the operational CO₂ emissions from fossil-fueled power plants, large-scale deployment of this technology will likely drive continued production and use of coal and gas. Even before fossil fuels are burned, extraction, transport, and processing generate substantial GHG emissions, particularly for gas. Therefore, in addition to perpetuating the fossil fuel industry, even 90% efficient CCS reduces only a fraction of the life cycle emissions from coal and gas.
Widespread deployment of CCS in the electricity sector could also delay or crowd out deployment of wind, solar, and geothermal energy, slowing the clean energy transition that is already underway. Beyond these risks, the three-decade-long failure of power plant CCS to make the transition from pilot-scale science and technology to large-scale commercial deployment reflects its systemic problems and limitations. Unlike wind and solar energy, which have seen costs decline rapidly with development and deployment, CCS on power plants shows little evidence of a learning curve. It remains very expensive and very energy-intensive. A large-scale CCS demonstration project can cost more than US$1 billion to build and, in addition to its operational costs, CCS consumes at least 15–25% of the energy that the plant could otherwise sell to customers. CCS-related energy requirements could mean that a power company would need to build an additional power plant to compensate for reduced electricity deliveries from every four of its power plants equipped with CCS.
Due to these high project risks and costs, as well as the lack of regulations and policies to require or support CCS on power plants, public and private investments in the technology have been falling. Despite all this, recent research shows that the vast majority of lobbying spending for government support of CCS comes from fossil fuel interests, which have publicly stated that they view the technology as a strategy to extend society’s use of fossil fuels. Finally, in contrast to most other climate solutions that provide other benefits to natural systems or human well-being, CCS on power plants does nothing to address or alleviate the current harm from toxic air pollution produced by fossil-fueled power plants.
Abdulla, A., Hanna, R., Schell, K. R., Babacan, O., & Victor, D. G. (2020). Explaining successful and failed investments in US carbon capture and storage using empirical and expert assessments. Environmental Research Letters, 16(1), 014036. Link to source: https://iopscience.iop.org/article/10.1088/1748-9326/abd19e?trk=public_post_comment-text
Caesary, D., Kim, H., & Nam, M. J. (2025). Cost effectiveness of carbon capture and storage based on probability estimation of social cost of carbon. Applied Energy, 377, 124542. Link to source: https://www.sciencedirect.com/science/article/abs/pii/S0306261924019251
Corcuera, E. G. T., & Petrakopoulou, F. (2025). Evaluating the impact of CO2 capture and storage on total efficiency: A lifecycle analysis. Cleaner Engineering and Technology, 101002. Evaluating the impact of CO2 capture and storage on total efficiency: A lifecycle analysis - ScienceDirect
Dabbs, B., Anchondo, C., & Marshall, C. (2023) The complete guide to CCS and the EPA power plant rule. Energywire, E&E News, May 10, 2023. The complete guide to CCS and the EPA power plant rule - E&E News by POLITICO
Drugman, D. (2023) Big Oil’s Been Secretly Validating Critics’ Concerns about Carbon Capture. DeSmog. Big Oil’s Been Secretly Validating Critics’ Concerns about Carbon Capture - DeSmog
Durmaz, T. (2018). The economics of CCS: Why have CCS technologies not had an international breakthrough?. Renewable and Sustainable Energy Reviews, 95, 328-340. The economics of CCS: Why have CCS technologies not had an international breakthrough? - ScienceDirect
Gibbons, B. (2024) In Illinois, a massive taxpayer-funded carbon capture project fails to capture about 90 percent of plant’s emissions. Oil and Gas Watch, Environmental Integrity Project. Link to source: https://news.oilandgaswatch.org/post/in-illinois-a-massive-taxpayer-funded-carbon-capture-project-fails-to-capture-about-90-percent-of-plants-emissions
Gonzales, V., Krupnick, A. and Dunlap, L. (2020) Carbon Capture and Storage 101. Resources for the Future. Link to source: https://media.rff.org/documents/CCS_101.pdf
Grubert, E., & Sawyer, F. (2023). US power sector carbon capture and storage under the Inflation Reduction Act could be costly with limited or negative abatement potential. Environmental Research: Infrastructure and Sustainability, 3(1), 015008. Link to source: https://iopscience.iop.org/article/10.1088/2634-4505/acbed9
Gulden, L. E., & Harvey, C. (2025). Tracing sources of funds used to lobby the US government about carbon capture, use, and storage. Environmental Science & Policy, 171, 104171. Link to source: https://www.sciencedirect.com/science/article/pii/S146290112500187X
Guo, J. X., & Huang, C. (2020). Feasible roadmap for CCS retrofit of coal-based power plants to reduce Chinese carbon emissions by 2050. Applied Energy, 259, 114112. Link to source: https://www.sciencedirect.com/science/article/abs/pii/S0306261919317994
Herzog, H. & Krol, A. (2025) Carbon Capture. MIT Climate Portal. “Carbon Capture” Carbon Capture | MIT Climate Portal
Herzog, H. & MIT Climate Portal Writing Team. (2024) If a fossil fuel power plant uses carbon capture and storage, what percent of the energy it makes goes to the CCS equipment? MIT Climate Portal. If a fossil fuel power plant uses carbon capture and storage, what percent of the energy it makes goes to the CCS equipment? | MIT Climate Portal
Hiar. C. (2023) Oil companies want to remove carbon from the air — using taxpayer dollars. Climatewire, E&E News, July, 13, 2023. Oil companies want to remove carbon from the air — using taxpayer dollars - E&E News by POLITICO
International Energy Agency (2020) The role of CCUS in low-carbon power systems. The role of CCUS in low-carbon power systems. subsection How carbon capture technologies support the power transition – The role of CCUS in low-carbon power systems – Analysis - IEA
International Energy Agency (2023). Emissions from Oil and Gas Operations in Net Zero Transitions: A World Energy Outlook Special Report on the Oil and Gas Industry and COP28. Link to source: https://iea.blob.core.windows.net/assets/2f65984e-73ee-40ba-a4d5-bb2e2c94cecb/EmissionsfromOilandGasOperationinNetZeroTransitions.pdf
International Energy Agency (2025) Global Energy Review 2025: CO2 Emissions. CO2 Emissions – Global Energy Review 2025 – Analysis - IEA
Jacobson, M. Z., Fu, D., Sambor, D. J., & Muhlbauer, A. (2025). Energy, health, and climate costs of carbon-capture and direct-air-capture versus 100%-wind-water-solar climate policies in 149 countries. Environmental Science & Technology, 59(6), 3034-3045. Energy, Health, and Climate Costs of Carbon-Capture and Direct-Air-Capture versus 100%-Wind-Water-Solar Climate Policies in 149 Countries | Environmental Science & Technology
Jacobson, M. Z. (2019). The health and climate impacts of carbon capture and direct air capture. Energy & Environmental Science, 12(12), 3567-3574. The health and climate impacts of carbon capture and direct air capture
Liu, S., Li, H., Zhang, K., & Lau, H. C. (2022). Techno-economic analysis of using carbon capture and storage (CCS) in decarbonizing China's coal-fired power plants. Journal of Cleaner Production, 351, 131384. Techno-economic analysis of using carbon capture and storage (CCS) in decarbonizing China's coal-fired power plants - ScienceDirect
Loria, P., & Bright, M. B. (2021). Lessons captured from 50 years of CCS projects. The Electricity Journal, 34(7), 106998. Link to source: https://www.sciencedirect.com/science/article/abs/pii/S1040619021000890
Ma, J., Li, L., Wang, H., Du, Y., Ma, J., Zhang, X., & Wang, Z. (2022). Carbon capture and storage: history and the road ahead. Engineering, 14, 33-43. Carbon Capture and Storage: History and the Road Ahead - ScienceDirect
Mackler, S., Fishman, X., & Broberg, D. (2021). A policy agenda for gigaton-scale carbon management. The Electricity Journal, 34(7), 106999. A policy agenda for gigaton-scale carbon management - ScienceDirect
National Energy Technology Laboratory. (2018). Carbon Capture and Storage Database (Washington, DC: U.S. Department of Energy). Link to source: https://netl.doe.gov/carbon-management/carbon-storage/worldwide-ccs-database
Osman, A. I., Hefny, M., Abdel Maksoud, M. I. A., Elgarahy, A. M., & Rooney, D. W. (2021). Recent advances in carbon capture storage and utilisation technologies: a review. Environmental Chemistry Letters, 19(2), 797-849. Recent advances in carbon capture storage and utilisation technologies: a review
Patel, S. (2024) Capturing Progress: The State of CCS in the Power Sector. POWER Magazine. Link to source: https://www.powermag.com/capturing-progress-the-state-of-ccs-in-the-power-sector/
Peridas, G., & Schmidt, B. M. (2021). The role of carbon capture and storage in the race to carbon neutrality. The Electricity Journal, 34(7), 106996. Link to source: https://www.sciencedirect.com/science/article/pii/S1040619021000877
Rathi, A. K. A., & Rathi, J. A. (2025). CO2 capture: a concise, comprehensive overview of recent research trends. Academia Environmental Sciences and Sustainability, 2(2). Rathi and Rathi 2025 CO2_capture_a_concise_comprehensive_overview.pdf
Scott, M. & Slavin, T. (2023) Fossil-fuel industry embrace raises alarm bells over direct air capture. Reuters, October 10, 2023. Fossil-fuel industry embrace raises alarm bells over direct air capture | Reuters
Singh, S. P., Ku, A. Y., Macdowell, N., & Cao, C. (2022). Profitability and the use of flexible CO2 capture and storage (CCS) in the transition to decarbonized electricity systems. International Journal of Greenhouse Gas Control, 120, 103767. Profitability and the use of flexible CO2 capture and storage (CCS) in the transition to decarbonized electricity systems - ScienceDirect
Stephens, J. C. (2014). Time to stop investing in carbon capture and storage and reduce government subsidies of fossil‐fuels. Wiley Interdisciplinary Reviews: Climate Change, 5(2), 169-173. Time to stop investing in carbon capture and storage and reduce government subsidies of fossil‐fuels - Stephens - 2014 - WIREs Climate Change - Wiley Online Library
Wang, N., Akimoto, K., & Nemet, G. F. (2021). What went wrong? Learning from three decades of carbon capture, utilization and sequestration (CCUS) pilot and demonstration projects. Energy Policy, 158, 112546. What went wrong? Learning from three decades of carbon capture, utilization and sequestration (CCUS) pilot and demonstration projects - ScienceDirect


Ocean alkalinity enhancement (OAE) increases the ocean’s natural ability to remove CO₂ from the air by increasing the alkalinity of ocean water. This carbon removal practice could be globally effective at removing CO₂ at the gigaton level annually and is currently being tested in field studies. Advantages of OAE include its ability to mitigate ocean acidification where it’s deployed and its scalability. Disadvantages include uncertainties surrounding OAEs’ global effectiveness and feasibility, potential impacts on marine life and humans, complex monitoring needed for verification, and potentially high costs, all of which need to be more closely studied. We will “Keep Watching” Deploy Ocean Alkalinity Enhancement until the technology advances and its risks, costs, and benefits become clearer.
Based on our analysis, OAE could be a promising carbon removal technique, but it is not ready for large-scale deployment until the risks, costs, and effectiveness become clearer. We will “Keep Watching” this potential climate solution.
| Plausible | Could it work? | Yes |
|---|---|---|
| Ready | Is it ready? | No |
| Evidence | Are there data to evaluate it? | Limited |
| Effective | Does it consistently work? | No |
| Impact | Is it big enough to matter? | Yes |
| Risk | Is it risky or harmful? | ? |
| Cost | Is it cheap? | ? |
OAE is the practice of adding alkalinity to seawater to increase the ocean’s ability to remove atmospheric CO₂. The addition of alkalinity through OAE mimics the natural process of weathering, or the physical and chemical breakdown of rocks. Rock weathering on land produces alkaline substances that eventually flow into the ocean through rivers and groundwater. This natural supply of alkalinity reduces ocean acidity, which affects the distribution of various carbon forms in the ocean. As alkalinity increases, CO₂ dissolved in seawater shifts toward more stable carbon forms, like bicarbonate and carbonate ions, that cannot exchange with air. This allows the ocean to remove more gaseous CO₂ from the atmosphere because the ocean and the atmosphere maintain a balance of CO₂ through gas movement at the sea surface. Most of the dissolved carbon in the ocean is bicarbonate and carbonate ions, which can persist in seawater for thousands of years. Under natural conditions, the ocean removes nearly 0.5 Gt of CO₂ annually. OAE generally relies on dissolving large amounts of ground-up rocks, either directly in the ocean or indirectly in water that is added to the ocean, to increase alkalinity and remove CO₂. This practice typically requires mining for alkaline rocks, though alkaline materials can also be sourced from waste by-products of other industries (e.g., steel slag, mine tailings) or commercially through human-made substances.
The science behind OAE is theoretically sound, and OAE is expected to result in durable storage over long time periods (>100 years). At scale, OAE could potentially remove over 1 Gt CO₂ /yr, but additional lab and field-based studies are needed to understand whether this approach is effective and safe. The majority of our understanding of OAE comes from models and laboratory experiments. However, when crushed minerals have been dispersed in field studies, the dissolution has not always occurred as expected. Several large-scale experimental trials are currently underway or planned, which will produce real-world data and inform monitoring and verification tactics needed to help refine and guide future implementation. These tests will also provide critical information on any ecological or community impacts. Various ways of implementing OAE are being developed, including ship-based dispersal, shoreline-based systems, and other approaches that leverage existing industrial waste streams or combine with other marine carbon dioxide removal (mCDR) techniques, such as electrochemical alkalinity generation.
OAE removes CO₂ from the atmosphere and stores it in the ocean as bicarbonate and carbonate ions, which are stable over long time periods. This means the CO₂ would be durably stored from the atmosphere for thousands of years. OAE could be scaled globally and can also mitigate local ocean acidification, a growing issue that threatens a range of marine ecosystems. Indeed, adding alkalinity to seawater has already been shown to mitigate ocean acidification in some coral reefs. Mitigating ocean acidification could also benefit fisheries and aquaculture, highlighting the potential for OAE to provide additional local benefits beyond carbon removal.
Several technical, environmental, and social concerns surround OAE. The effectiveness could be limited by real-world conditions that either transport the alkaline materials away from the ocean’s surface before CO₂ can be absorbed or result in unexpected chemical reactions or biological uptake of the added alkalinity. Measuring and verifying the amount of CO₂ permanently stored using OAE is also challenging and will rely on a combination of field data and complex numerical models, which will require significant effort to collect and develop. Beyond these technical challenges, OAE poses potential environmental risks on land and in the ocean. On land, OAE could require an expansion of mining that rivals the cement industry, which could have negative environmental impacts on human and ecosystem health. In the ocean, increased alkalinity and the potential release of metals from the source rocks could negatively affect some marine life, but our understanding of the effects on individual species and food webs is limited. OAE could also interfere with existing ocean uses (e.g., fisheries, recreation) in some places and could have other unintended consequences as well. For instance, research suggests that OAE reduces natural alkalinity production in some ocean areas. In addition, OAE faces several social challenges. To be successful, mCDR approaches, like OAE, will require rapid, meaningful, and just community engagement. Public concerns about OAE have already led to a pilot project cancellation, highlighting the importance of public perception for OAE feasibility. It is also unclear if OAE can be scaled globally at reasonable costs, with current estimates highly variable but generally over US$100/t CO₂. Lastly, acquiring and dispersing sufficient alkaline materials could be challenging at scale, particularly because some materials are currently energy-intensive to source, transport, and/or produce.
Albright, R., Caldeira, L., Hosfelt, J., Kwiatkowski, L., Maclaren, J. K., Mason, B. M., ... & Caldeira, K. (2016). Reversal of ocean acidification enhances net coral reef calcification. Nature, 531(7594), 362-365. Link to source: https://doi.org/10.1038/nature17155
Bach, L. T. (2024). The additionality problem of ocean alkalinity enhancement. Biogeosciences, 21(1), 261-277. Link to source: https://doi.org/10.5194/bg-21-261-2024
Bach, L. T., Gill, S. J., Rickaby, R. E., Gore, S., & Renforth, P. (2019). CO2 removal with enhanced weathering and ocean alkalinity enhancement: potential risks and co-benefits for marine pelagic ecosystems. Frontiers in Climate, 1, 7. Link to source: https://doi.org/10.3389/fclim.2019.00007
Bertram, C., & Merk, C. (2020). Public perceptions of ocean-based carbon dioxide removal: the nature-engineering divide?. Frontiers in Climate, 2, 594194. Link to source: https://doi.org/10.3389/fclim.2020.594194
(carbon)plan. Introduction to Ocean Alkalinity Enhancement: Link to source: https://carbonplan.org/research/oae-efficiency-explainer
Carbon Herald. (2025, April 11). Planetary Technologies cancels its mCDR project in Cornwall. Link to source: https://carbonherald.com/planetary-technologies-cancels-its-mcdr-project-in-cornwall/
Doney, S. C., Wolfe, W. H., McKee, D. C., & Fuhrman, J. G. (2024). The science, engineering, and validation of marine carbon dioxide removal and storage. Annual Review of Marine Science, 17. Link to source: https://doi.org/10.1146/annurev-marine-040523-014702
Doney, S. C., Fabry, V. J., Feely, R. A., & Kleypas, J. A. (2009). Ocean acidification: the other CO2 problem. Annual Review of Marine Science, 1(1), 169-192. Link to source: https://doi.org/10.1146/annurev.marine.010908.163834
EGU Biogeosciences. Special Issue: Environmental impacts of ocean alkalinity enhancement. Link to source: https://bg.copernicus.org/articles/special_issue1246.html
Gattuso, J. P., Magnan, A. K., Bopp, L., Cheung, W. W., Duarte, C. M., Hinkel, J., ... & Rau, G. H. (2018). Ocean solutions to address climate change and its effects on marine ecosystems. Frontiers in Marine Science, 5, 337. Link to source: https://doi.org/10.3389/fmars.2018.00337
Oschlies, A., Stevenson, A., Bach, L. T., Fennel, K., Rickaby, R. E. M., Satterfield, T., Webb, R., and Gattuso, J.-P. (2023). Guide to Best Practices in Ocean Alkalinity Enhancement Research, Copernicus Publications, State of the Planet, 2-oae2023. Link to source: https://doi.org/10.5194/sp-2-oae2023
Hartmann, J., Suitner, N., Lim, C., Schneider, J., Marín-Samper, L., Arístegui, J., ... & Riebesell, U. (2022). Stability of alkalinity in ocean alkalinity enhancement (OAE) approaches–consequences for durability of CO 2 storage. Biogeosciences Discussions, 2022, 1-29. Link to source: https://doi.org/10.5194/bg-20-781-2023
Hartmann, J., West, A. J., Renforth, P., Köhler, P., De La Rocha, C. L., Wolf‐Gladrow, D. A., ... & Scheffran, J. (2013). Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Reviews of Geophysics, 51(2), 113-149. Link to source: https://doi.org/10.1002/rog.20004
He, J., & Tyka, M. D. (2023). Limits and CO2 equilibration of near-coast alkalinity enhancement. Biogeosciences, 20(1), 27-43. Link to source: https://doi.org/10.5194/bg-20-27-2023
National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Ocean Studies Board; Committee on A Research Strategy for Ocean-based Carbon Dioxide Removal and Sequestration. A Research Strategy for Ocean-based Carbon Dioxide Removal and Sequestration. Washington (DC): National Academies Press (US); 2021 Dec 8. 7, Ocean Alkalinity Enhancement. Link to source: https://www.ncbi.nlm.nih.gov/books/NBK580052/
Ocean Visions: Link to source: https://oceanvisions.org/ocean-alkalinity-enhancement/
Palmiéri, J. and Yool, A., 2024. Global‐scale evaluation of coastal ocean alkalinity enhancement in a fully coupled Earth system model. Earth's Future, 12(3), p.e2023EF004018. Link to source: https://doi.org/10.1029/2023EF004018
Renforth, P., & Henderson, G. (2017). Assessing ocean alkalinity for carbon sequestration. Reviews of Geophysics, 55(3), 636-674. Link to source: https://doi.org/10.1002/2016RG000533
Satterfield, T., Nawaz, S., & Boettcher, M. (2023). Social considerations and best practices for engaging publics on ocean alkalinity enhancement. State of the Planet Discussions, 2023, 1-39. Link to source: https://doi.org/10.5194/sp-2-oae2023-11-2023
Webb, R. M., Silverman-Roati, K., & Gerrard, M. B. (2021). Removing Carbon Dioxide Through Ocean Alkalinity Enhancement: Legal Challenges and Opportunities. Link to source: https://scholarship.law.columbia.edu/faculty_scholarship/2981
Zhuang, W., Zhu, T., Li, F., Queiroz, H. M., Yan, Q., Zhao, X., & Liu, J. (2025). Potential Environmental Impacts and Management Strategies for Metal Release during Ocean Alkalinity Enhancement Using Olivine. Environmental Science & Technology, 59(2), 1091-1099. Link to source: https://doi.org/10.1021/acs.est.4c10705
Zhou, M., Tyka, M. D., Ho, D. T., Yankovsky, E., Bachman, S., Nicholas, T., ... & Long, M. C. (2024). Mapping the global variation in the efficiency of ocean alkalinity enhancement for carbon dioxide removal. Nature Climate Change, 15(1), 59-65. Link to source: https://doi.org/10.1038/s41558-024-02179-9

Enhanced rock weathering removes CO₂ from the air by accelerating the natural chemical and physical breakdown of certain rocks. This carbon removal practice can be effective and has been deployed in pilot and small-scale commercial projects. Advantages include its reliance on a natural process (geological weathering), its potential for large-scale deployment on land or in the ocean, and its potential to improve soil conditions and crop yields. Disadvantages of enhanced rock weathering include unpredictable effectiveness for carbon removal, complex monitoring and measurement requirements, and high costs. We will “Keep Watching” Enhanced Rock Weathering, but it is not yet ready for large-scale deployment as a climate solution.
Based on our analysis, enhanced rock weathering is a promising carbon removal technique, but it is not ready for large-scale deployment. We will “Keep Watching” this potential climate solution.
| Plausible | Could it work? | Yes |
|---|---|---|
| Ready | Is it ready? | Yes |
| Evidence | Are there data to evaluate it? | Yes |
| Effective | Does it consistently work? | No |
| Impact | Is it big enough to matter? | Yes |
| Risk | Is it risky or harmful? | No |
| Cost | Is it cheap? | No |
Enhanced rock weathering is a practice that removes CO₂ from the atmosphere by accelerating the natural chemical and physical breakdown, or weathering, of rocks such as basalt, olivine, or limestone. This is typically achieved by crushing the rocks into dust or sand-sized particles to increase their surface area before applying them to croplands, beaches, or directly into the ocean, the latter of which is also a form of carbon removal known as ocean alkalinity enhancement. During weathering, the rock surface chemically reacts with atmospheric CO₂ that is dissolved in rain or ocean water. This reaction produces bicarbonate ions containing the carbon from the captured CO₂ and positively charged cations, such as magnesium or calcium, depending on the type of rock. For land-based enhanced rock weathering, the bicarbonate needs to be flushed out to the ocean, where it is stable and can be securely stored for thousands of years.
The basic idea of enhanced rock weathering is scientifically and geologically sound. Its effectiveness in converting atmospheric CO₂ into bicarbonate has been demonstrated in laboratory and field trials for several rock types and application sites. There are currently numerous research and demonstration projects underway. More than a dozen companies are selling enhanced rock weathering-based carbon removal credits, with nearly 10,000 t CO₂ reported to have been removed as of early 2025.
Enhanced rock weathering has several features that improve the likelihood that it can be scaled up to remove and store globally meaningful amounts of atmospheric CO₂ (>0.1 Gt CO₂/yr). Since enhanced rock weathering utilizes a natural process – mineralization – it does not need to be combined with other technologies to capture CO₂ from the air or durably store it. Moreover, it does not require external energy for the carbon capture and storage process, although it does use energy and generate emissions from the mining, crushing, transport, and deployment of the crushed rock. Suitable rock types, such as basalt, which is widely used in construction, paving, and concrete, are common and often locally available. Globally, there are large areas of land and ocean surface on which enhanced rock weathering could be deployed, including on croplands where current agricultural practices often already include regular application of soil amendments. A recent study suggested that extensive deployment of enhanced rock weathering on U.S. agricultural lands could sequester 0.16–0.30 Gt CO₂/yr by 2050. Other studies have shown that the application of crushed rock to croplands for enhanced rock weathering can improve soil pH, provide essential soil nutrients, and improve crop yields.
There are numerous challenges for enhanced rock weathering, as well as potential risks and adverse impacts from its large-scale deployment. Numerous studies on both land- and ocean-based enhanced rock weathering have shown that the amounts of atmospheric CO₂ converted into bicarbonate are highly variable, dependent on rock type, soil type, application rates, and other variables, and are therefore difficult to accurately predict and model. This makes measurement, reporting, and verification of the amount of CO₂ captured and stored, which is essential for the carbon market, reliant on extensive and expensive field measurements and customized models. There are also concerns about the harmful impacts of heavy metals, like nickel or chromium, that can be released during weathering, as well as other ecological impacts and environmental justice concerns, particularly for crushed rock deployed on beaches or in the ocean. Finally, costs for deployment and the purchase of enhanced rock weathering-based carbon credits are relatively high (>US$200–US$500/t CO₂ removed) and will likely remain high if verification continues to depend on large numbers of field measurements and carbon removal cannot be easily modeled. There is a general consensus in the scientific community that the current knowledge base is not sufficient to reliably or accurately quantify the CO₂ captured and stored by most land- or ocean-based enhanced rock weathering deployments.
Bach, L. T., Gill, S. J., Rickaby, R. A., Gore, S., & Renforth, P. (2019). CO2 removal with enhanced weathering and ocean alkalinity enhancement: potential risks and co-benefits for marine pelagic ecosystems. Frontiers in Climate 1(7). Link to source: https://doi.org/10.3389/fclim.2019.00007
Beerling, D. J. et al. (2025). Transforming US agriculture for carbon removal with enhanced weathering. Nature 638, 425–434. Link to source: https://doi.org/10.1038/s41586-024-08429-2
CDR.fyi. Leaderboards. (2025). Leaderboards. Retrieved from CDR.fyi website: Link to source: https://www.cdr.fyi/leaderboards
Cong, L., Lu, S., Jiang, P., Zheng, T., Yu, Z., & Lü, X. (2024). CO₂ sequestration and soil improvement in enhanced rock weathering: A review from an experimental perspective. Greenhouse. Gas. Sci. Technol., 14, 1122–1138. Link to source: https://doi.org/10.1002/ghg.2313
Geerts, L. J., Hylén, A., & Meysman, F. J. (2025). Review and syntheses: Ocean alkalinity enhancement and carbon dioxide removal through marine enhanced rock weathering using olivine. Biogeosciences 22(2), 355–384. Link to source: https://doi.org/10.5194/bg-22-355-2025
Höglund, R. (2025). Buyers of Enhanced Rock Weathering credits need to ask for the right type of MRV. Milkywire. Link to source: https://www.milkywire.com/articles/buyers-of-enhanced-rock-weathering-credits-need-to-ask-for-the-right-type-of-mrv
Jagoutz, O. & Krol, A. (2023). Enhanced Rock Weathering. MIT Climate Portal. Link to source: https://climate.mit.edu/explainers/enhanced-rock-weathering
Jeswani, H. K., Saharudin, D. M., & Azapagic, A. (2022). Environmental sustainability of negative emissions technologies: A review. Sustainable Production and Consumption 33, 608–635. Link to source: https://doi.org/10.1016/j.spc.2022.06.028
Jones, W., Bower, G., Pastorek, N., King, B., Larsen, J., Houser, T., Dasari, N., & McCusker, K. (2024). The landscape of carbon dioxide removal and US policies to scale solutions. Link to source: https://rhg.com/wp-content/uploads/2024/04/The-Landscape-of-Carbon-Dioxide-Removal-and-US-Policies-to-Scale-Solutions.pdf
Morris, A. (2024). Testing limestone’s ability to capture carbon from air. Northwestern Now. Link to source: https://news.northwestern.edu/stories/2024/11/northwestern-scientists-test-limestones-ability-to-capture-carbon-from-air/
OPIS & CDR.fyi. (2025). Bridging the gap: Durable CDR market pricing survey. Link to source: https://www.cdr.fyi/reports/pricing-survey-jan-2025.pdf
Taylor, L. et al. (2016). Enhanced weathering strategies for stabilizing climate and averting ocean acidification. Nature Climate Change 6, 402–406. Link to source: https://doi.org/10.1038/nclimate2882
Join the 85,000+ subscribers discovering how to drive meaningful climate action around the world! Every other week, you'll get expert insights, cutting-edge research, and inspiring stories.